Radiocarbon Detection / Overview
Radiocarbon Measurement
Carbon isotopes are present in every living thing, the ¹²C isotope is stable and is the most present ~99%, ¹³C is approximately 1%,
¹⁴C (radiocarbon) is present in very small concentrations ~10-¹² (1ppt), is produced by cosmic rays in the upper atmosphere, due to its radioactive half-life of 5700 years, after the death of any living thing, radiocarbon works as a natural clock for very long times.
Radiocarbon is a label provided by nature for the biogenic origin of any carbon-containing material.
Radiocarbon is no longer present in biogenic products of fossil origin (like coal, coal products, natural gas, derived gas, crude oil, petroleum) because it has completely decayed.
Radiocarbon can be used to discriminate the fossil fraction in materials and gases.
Finally, being naturally present in animal physiology, radiocarbon it is fully tolerated in metabolic processes, for this reason it can also be used for pharmacology studies.
Radiocarbon-enriched samples are used as radiolabels for the study of metabolic processes in drugs.
SCAR technique
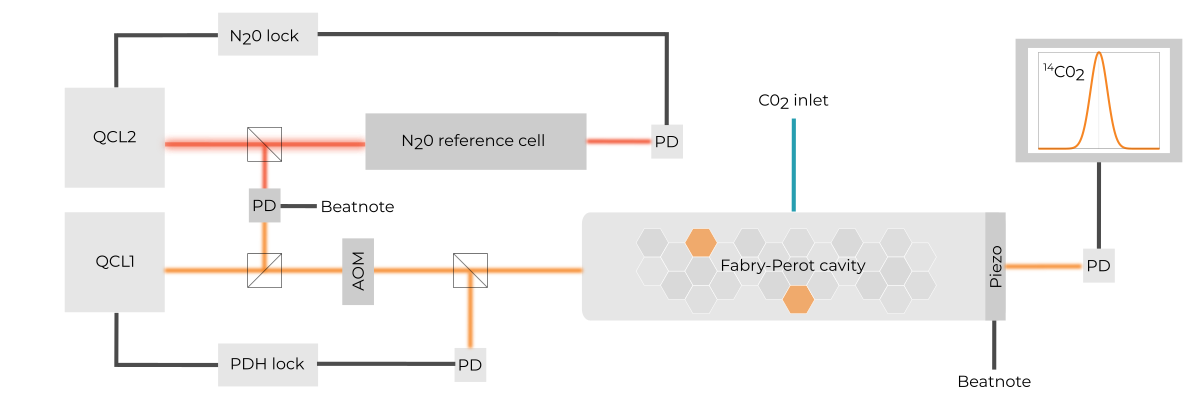
ppqSense developed a laser spectroscopy technique that is sensitive enough to detect radiocarbon dioxide molecules at very low mole fractions with an all-optical setup, that is more compact and less expensive than AMS and much “cleaner” than LSC, the two currently most widely used techniques for measuring radiocarbon.
The new approach, named Saturated-absorption CAvity Ring-down (SCAR), makes use of molecular absorption saturation to enhance the sensitivity with respect to conventional cavity ring-down spectroscopy.
By combining SCAR with narrow-linewidth mid-IR quantum cascade lasers (QCLs) and our state of the art electronics we can achieve an unprecedented limit in trace gas detection, down to a few parts-per-quadrillion, ppq, (10-¹⁵) mole fraction.
C14-SCAR analyzes the CO2 gas produced by burning the sample and retrieves the mole fraction of 14C by measuring the spectral area of a given molecular transition of 14CO2 molecule.
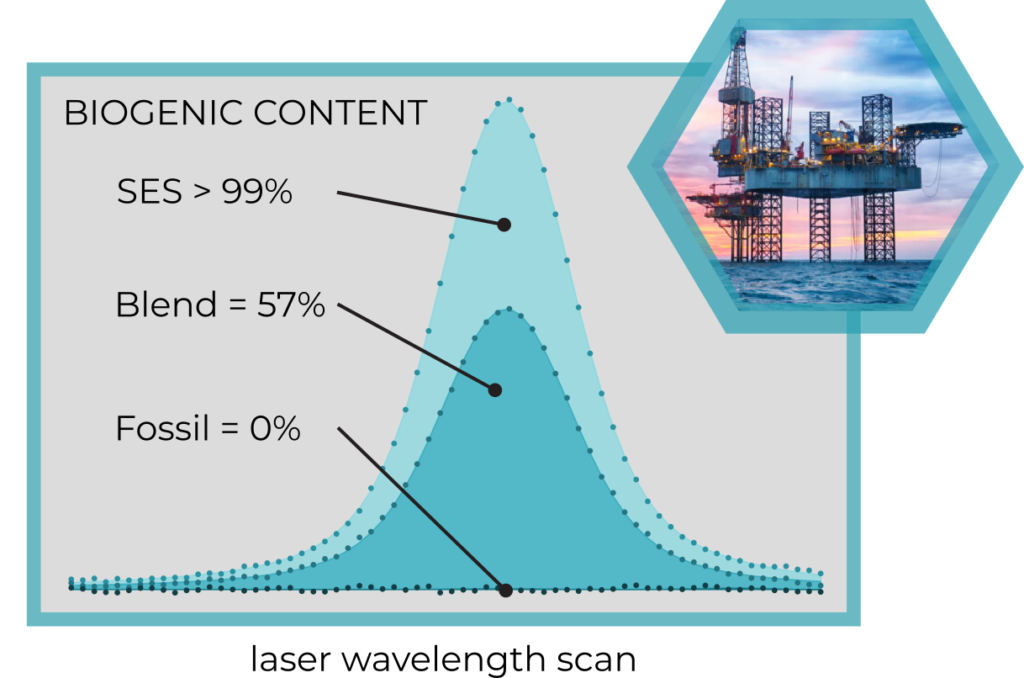
Biogenic content on three types of fuels
SES biofuel produced from seaweed confirms its
biogenic origin.
A blend of fosiil and bio-fules can be precisely
characterized.
Pure fossil fuel shows a 0% biogenic component.
A natural leather treated with synthetic leather tanning
substances shows an overall biogenic content much
lower than the neatural abundance.
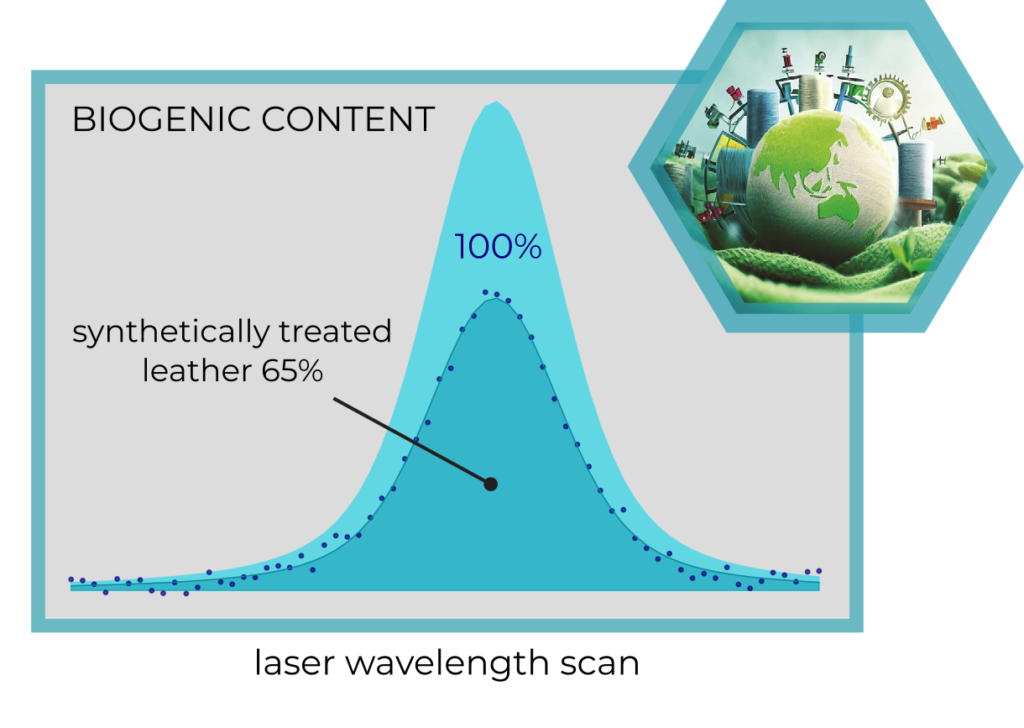
Effects of synthetic treatment on natural leathers
A natural leather treated with synthetic leather tanning substances shows an overall biogenic content much lower than the neatural abundance.
If the sample is taken from a modern living being, the measured 14C mole fraction will be close to the so-called natural abundance or Modern Carbon (MC) mole fraction. This corresponds to 100 percent of Modern Carbon, i.e. 100 pMC.
A similar measurement taken on a sample containing only fossil carbon will not show any signal corresponding to the 14CO2 transition, since no 14C is present: this corresponds to 0 percent of Modern Carbon, 0 pMC.
C14-SCAR Instrument

C14-SCAR is a high-precision, laser-based, table-top ¹⁴CO₂ analyzer. Thanks to its new technology it allows to take a radiocarbon measurements in your laboratory in a simple and reliable way.
C14-SCAR has outstanding features compared to other instruments for radiocarbon measurement.
Accelerator Mass Spectrometry (AMS) detects isotope ratio of ¹²C, ¹³C, ¹⁴C ions, has an excellent sensitivity, requires a small carbon mass and provide short measurement times. However, AMS requires huge, expensive and high-maintenance experimental facilities. Its economic and energy costs make only ~20 instruments available in Europe.
Liquid Scintillation Counting (LSC) uses β-decay counting, is cheap but but with insufficient precision for monitoring applications, thus requiring large sample amounts, high isotope dose and scintillation cocktails must be disposed off in a special way.
–
SCAR
AMS
LSC
SIZE
![]()
![]()
![]() –
–![]()
![]()
![]()
![]() –
–
WEIGHT
![]()
![]()
![]()
![]()
![]()
![]()
![]() –
–![]()
MINIMUM SAMPLE NEEDS
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
PRECISION MEASUREMENT
![]()
![]()
![]()
![]()
![]()
![]()
DYNAMIC RANGE
![]()
![]()
![]()
![]()
![]()
![]()
COST [€]
![]()
![]()
![]()
![]()
![]()
![]()
SAMPLE HANDLING
![]()
![]()
![]()
![]()
![]()
![]()
SIZE
SCAR
![]()
![]()
AMS
![]()
![]()
![]()
LSC
![]()
![]()
WEIGHT
SCAR
![]()
![]()
AMS
![]()
![]()
![]()
![]()
LSC
![]()
![]()
MINIMUM SAMPLE NEEDS
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
![]()
![]()
PRECISION
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
DYNAMIC RANGE
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
SAMPLE HANDLING
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
COST [€]
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
C14 SCAR
Radiocarbon Measurement

Carbon isotopes are present in every living thing, the ¹²C isotope is stable and is the most present ~99%, ¹³C is approximately 1%,
¹⁴C (radiocarbon) is present in very small concentrations ~10-¹² (1ppt), is produced by cosmic rays in the upper atmosphere, due to its radioactive half-life of 5700 years, after the death of any living thing, radiocarbon works as a natural clock for very long times.
Radiocarbon is a label provided by nature for the biogenic origin of any carbon-containing material.
Radiocarbon is no longer present in biogenic products of fossil origin (like coal, coal products, natural gas, derived gas, crude oil, petroleum) because it has completely decayed.
Radiocarbon can be used to discriminate the fossil fraction in materials and gases.
Finally, being naturally present in animal physiology, radiocarbon it is fully tolerated in metabolic processes, for this reason it can also be used for pharmacology studies.
Radiocarbon-enriched samples are used as radiolabels for the study of metabolic processes in drugs.
SCAR technique
ppqSense developed a laser spectroscopy technique that is sensitive enough to detect radiocarbon dioxide molecules at very low mole fractions with an all-optical setup, that is more compact and less expensive than AMS and much “cleaner” than LSC, the two currently most widely used techniques for measuring radiocarbon.
The new approach, named Saturated-absorption CAvity Ring-down (SCAR), makes use of molecular absorption saturation to enhance the sensitivity with respect to conventional cavity ring-down spectroscopy.
By combining SCAR with narrow-linewidth mid-IR quantum cascade lasers (QCLs) and our state of the art electronics we can achieve an unprecedented limit in trace gas detection, down to a few parts-per-quadrillion, ppq, (10-¹⁵) mole fraction.
C14-SCAR analyzes the CO2 gas produced by burning the sample and retrieves the mole fraction of 14C by measuring the spectral area of a given molecular transition of 14CO2 molecule.

Biogenic content on three types of fuels
SES biofuel produced from seaweed confirms its
biogenic origin.
A blend of fosiil and bio-fules can be precisely
characterized.
Pure fossil fuel shows a 0% biogenic component.
A natural leather treated with synthetic leather tanning
substances shows an overall biogenic content much
lower than the neatural abundance.

Effects of synthetic treatment on natural leathers
A natural leather treated with synthetic leather tanning substances shows an overall biogenic content much lower than the neatural abundance.
If the sample is taken from a modern living being, the measured 14C mole fraction will be close to the so-called natural abundance or Modern Carbon (MC) mole fraction. This corresponds to 100 percent of Modern Carbon, i.e. 100 pMC.
A similar measurement taken on a sample containing only fossil carbon will not show any signal corresponding to the 14CO2 transition, since no 14C is present: this corresponds to 0 percent of Modern Carbon, 0 pMC.
C14-SCAR Instrument

C14-SCAR is a high-precision, laser-based, table-top ¹⁴CO₂ analyzer. Thanks to its new technology it allows to take a radiocarbon measurements in your laboratory in a simple and reliable way.
C14-SCAR has outstanding features compared to other instruments for radiocarbon measurement.
Accelerator Mass Spectrometry (AMS) detects isotope ratio of ¹²C, ¹³C, ¹⁴C ions, has an excellent sensitivity, requires a small carbon mass and provide short measurement times. However, AMS requires huge, expensive and high-maintenance experimental facilities. Its economic and energy costs make only ~20 instruments available in Europe.
Liquid Scintillation Counting (LSC) uses β-decay counting, is cheap but but with insufficient precision for monitoring applications, thus requiring large sample amounts, high isotope dose and scintillation cocktails must be disposed off in a special way.
–
SCAR
AMS
LSC
SIZE
![]()
![]()
![]() –
–![]()
![]()
![]()
![]() –
–
WEIGHT
![]()
![]()
![]()
![]()
![]()
![]()
![]() –
–![]()
MINIMUM SAMPLE NEEDS
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
PRECISION MEASUREMENT
![]()
![]()
![]()
![]()
![]()
![]()
DYNAMIC RANGE
![]()
![]()
![]()
![]()
![]()
![]()
COST [€]
![]()
![]()
![]()
![]()
![]()
![]()
SAMPLE HANDLING
![]()
![]()
![]()
![]()
![]()
![]()
SIZE
SCAR
![]()
![]()
AMS
![]()
![]()
![]()
LSC
![]()
![]()
WEIGHT
SCAR
![]()
![]()
AMS
![]()
![]()
![]()
![]()
LSC
![]()
![]()
MINIMUM SAMPLE NEEDS
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
![]()
![]()
PRECISION
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
DYNAMIC RANGE
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
SAMPLE HANDLING
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()
COST [€]
SCAR
![]()
![]()
AMS
![]()
![]()
LSC
![]()
![]()